how do you determine the mass of an atom|calculate atomic weight : Tuguegarao Using the periodic table, we see the atomic mass of nitrogen is 14.01 amu or 14.01 g/mol. 2. Add up the number of neutrons and protons in a single atom. If the mass .
web1XQQ adalah situs judi slot online terpercaya dan aman yang menawarkan pengalaman bermain yang luar biasa. Dengan berbagai bonus dan permainan yang ditawarkan, ini .
0 · how to find grams in an atom
1 · how to calculate mass number
2 · formula to calculate atomic mass
3 · formula of average atomic mass
4 · calculating the atomic mass
5 · calculate the atomic weight of an element
6 · calculate atomic weight
7 · atomic mass calculation example
8 · More
Juwa brings you the latest and best games. Try your luck on the slots, test your skill at fish games. Whatever your pleasure you will find it on Juwa, backed by the world-class .
how do you determine the mass of an atom*******To calculate the atomic mass of a single atom of an element, add up the mass of protons and neutrons. Example: Find the .how do you determine the mass of an atomSince the combined masses of protons and neutrons account for almost all the mass of the given atom, the atomic mass of a single atom can be calculated by adding the total . The atomic mass is the mass of an atom, including its protons, neutrons, and electrons. However, because an electron is 1,836 times less massive than a proton, . Look up the atomic mass of silver. It is 107.89 grams per mole (g/mol). Use Avogadro’s number. It is approximately 6.022×10 23. Divide the atomic mass by .
The mass of one atom is usually expressed in atomic mass units (amu), which is referred to as the atomic mass. An amu is defined as exactly 1 / 12 of the mass of a carbon-12 atom and is equal to 1.6605 × 10 −24 g. . Using the periodic table, we see the atomic mass of nitrogen is 14.01 amu or 14.01 g/mol. 2. Add up the number of neutrons and protons in a single atom. If the mass .Together, the number of protons and the number of neutrons determine an element’s mass number: mass number = protons + neutrons. If you want to calculate how many neutrons an atom has, you can simply subtract . Figure 3.4.1 3.4. 1: The social security number subatomic-the proton. Since atoms are neutral, the number of electrons in an atom is equal to the number of protons. Hydrogen atoms all have one electron . The atomic mass unit (abbreviated u, altho ugh amu is a lso used) is defined as 1/12 of the mass of a 12C atom: 1 u = 1 12 the mass of 12Catom (2.6.1) (2.6.1) 1 u = 1 12 the mass of 12 C a t o m. It is equal to 1.661 × 10 −24 g. Masses of other atoms are expressed with respect to the atomic mass unit. Since the vast majority of an atom’s mass is found its protons and neutrons, subtracting the number of protons (i.e. the atomic number) from the atomic mass will give you the calculated . The atomic mass unit (abbreviated u, altho ugh amu is a lso used) is defined as 1/12 of the mass of a 12C atom: 1 u = 1 12 the mass of 12Catom (2.6.1) (2.6.1) 1 u = 1 12 the mass of 12 C a t o m. It is equal to 1.661 × 10 −24 g. Masses of other atoms are expressed with respect to the atomic mass unit.
Alternatively, you can also calculate the atomic number, atomic mass, and charge. Choose your element. Let's assume that it is the sulfide anion. Find the numbers of protons, neutrons, and electrons. They are equal to 16, 16, and 18, respectively. Calculate atomic number, atomic mass, and charge by using mathematical expressions (4-6): Z = . The mass of an atom consists of the mass of the nucleus plus that of the electrons. That means the atomic mass unit is not exactly the same as the mass of the proton or neutron. . Since it is the electrons that determine how one atom interacts with another, in the end it is the number of protons in the nucleus that determines the .
Make sure that you round the atomic mass to the nearest whole number. For example, the atomic mass of boron is 10.811, but you can just round the atomic mass up to 11. 6. Subtract the atomic number from the atomic mass. To find the number of neutrons, you will need to subtract the atomic number from the atomic mass.calculate atomic weight The number is the atomic mass. Subtract the atomic number of the element from the atomic mass of the isotope, and the result is the number of neutrons in the nucleus of that isotope. In the case of C-14, the atomic number of carbon is 6, so there must be 8 neutrons in the nucleus. That's two more than the more common, balanced .
For glucose, C 6 H 12 O 6, the molar mass of each element is: carbon, 12.0107 x 6 = 72.0642 g/mol; hydrogen, 1.007 x 12 = 12.084 g/mol; and oxygen, 15.9994 x 6 = 95.9964 g/mol. 4. Add the molar masses of each element in the compound. This determines the molar mass for the entire compound. Take the products you obtained in . 3. Multiply the volume and density together. Multiply your two numbers together, and you'll know the mass of your object. [1] Keep track of the units as you do this, and you'll see that you .

Now, because the atom has 53 electrons, it must also have 53 protons, and to find the number of neutrons we subtract this from the mass number. # n = A – # p = 127 – 53 = 74 neutrons. To summarize, you need to remember these relationships between the atomic mass, the number of protons, neutrons, and electrons: If an atom loses or gains electrons, it becomes an ion, which means that it is an electrically charged atom. 4. Calculate the number of neutrons. Now you know that atomic number = number .So once again for protons, we look at the atomic number, that's 92. So there must be 92 protons. In a neutral atom, the number of electrons is equal to the number of protons. So there are 92 electrons and then finally, to figure out the number of neutrons, we subtract this number from the mass number. So we just need to do 235 minus 92.
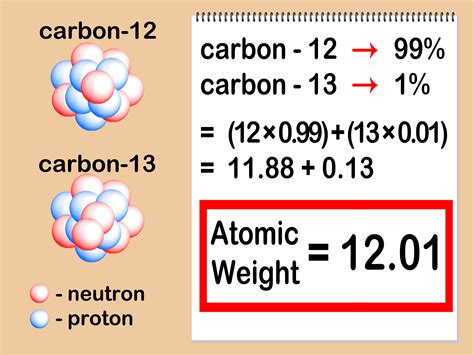
A mass spectrometer ionizes atoms and molecules with a high-energy electron beam and then deflects the ions through a magnetic field based on their mass-to-charge ratios ( m / z. . ). The mass spectrum of a sample shows the relative abundances of the ions on the y-axis and their m / z. . ratios on the x-axis. If z = 1.how do you determine the mass of an atom calculate atomic weight1. Because most elements exist as mixtures of several stable isotopes, the atomic mass of an element is defined as the weighted average of the masses of the isotopes. For example, naturally occurring carbon is largely a mixture of two isotopes: 98.89% 12 C (mass = 12 amu by definition) and 1.11% 13 C (mass = 13.003355 amu).
Nuclear binding energy is the energy required to split an atom’s nucleus into protons and neutrons. Mass defect is the difference between the predicted mass and the actual mass of an atom's nucleus. The binding energy of a system can appear as extra mass, which accounts for this difference. Created by Jay. To calculate atomic mass, start by finding the atomic number of the element, which is the number above the element on the periodic table. Next, find the number of neutrons in the nucleus by subtracting . To calculate the atomic mass of a single atom of an element, add up the mass of protons and neutrons. Example: Find the atomic mass of an isotope of carbon that has 7 neutrons . You can see from the periodic table that carbon has an atomic number of 6, which is its number of protons.How to Find Atomic Mass. To find Atomic mass, it is important to understand whether the given sample is an atom, a sample containing isotopes of the atom at a given ratio, or if it is a natural sample of the element. This atomic mass calculator shows you how to find the atomic mass of an atom using the atomic mass formula and explains the atomic mass definition. It also describes the atomic mass unit and explores why an atom needs neutrons.
Have you ever wondered about the mass of a single atom? It’s easy to calculate! All you need is the relative atomic mass (atomic weight) of the element and Avogadro’s number. Here is how you calculate the mass of a single atom and how you extend the same principle to find mass of a single molecule. mass of single atom = .
Atomic mass is the mass of a single atom. This tutorial shows the steps needed to calculate atomic mass depending on the type of problem you are working.
Together, the number of protons and the number of neutrons determine an element’s mass number: mass number = protons + neutrons. If you want to calculate how many neutrons an atom has, you can simply subtract the number of .Calculate average atomic mass and isotopic abundance. Define the amount unit mole and the related quantity Avogadro’s number. Explain the relation between mass, moles, and numbers of atoms or molecules, and perform calculations deriving .
web16 de set. de 2023 · Confira o resultado da Mega Sena 2633 do dia 16 de setembro de 2023, sábado, com o prêmio de hoje estimado em R$ 9.000.000,00 (nove milhões de .
how do you determine the mass of an atom|calculate atomic weight